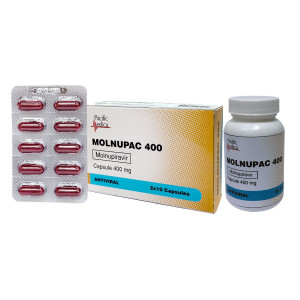
MOLNUPAC 400mg
- Category: ပိုးသတ်ဆေးများ
- UOM: Box of 2x10's Capsule
69,180 Kyats
Molnupac 400mg (Online အော်ဒါများကို ပို.ဆောင်ချိန် ၃ရက်မှ ၅ရက်ခန်.ကြာမြင့်နိုင်ပါတယ်)
This medicine is prescription only medicine (POM ) and required under medical supervision .For the order request process, you need to attach the doctor's prescription.
ဤဆေးသည် ဆရာဝန်ညွှန်ကြားချက်နှင့်သာသောက်သုံးရမည့်ဆေးဖြစ်ပြီး ဆရာဝန်ညွှန်ကြားချက်လိုပါသည်။ အော်ဒါမှာယူမှုအတွက် ဆရာဝန်ဆေးညွှန်းစာကို Order request အဆင့်တွင် attach တွဲပေးရန်လိုပါသည်။
REMARK : Online order delivery may take from 3 to 5 days depend on area. For some areas in Yangon region such as North Dagon, South Dagon , Dagon Seikkan , Hlaing Thar Yar, Shwe Pyi Thar , Thanlyin , delivery will be around 1 week.
(မှတ်ချက် : အွန်လိုင်းမှတဆင့်မှာယူမှုများအတွက် အခမဲ့ပို့ဆောင်မှုမှာ ၃ ရက်မှ ၅ ရက်အတွင်းကြာမြင့်နိုင်ပါသည်။ အချို့ ဧရိယာများဖြစ်သည့် မြောက်ဒဂုံ ၊ တောင်ဒဂုံ ၊ ဒဂုံဆိပ်ကမ်း ၊ လှိုင်သာယာ ၊ ရွှေပြည်သာ ၊ သံလျှင် စသည့်မြို့များမှာမှာ တစ်ပတ်တရက်သာ ပို့ဆောင်သောကြောင့် ၁ ပတ်ခန့်ကြာမြင့်နိုင်ပါသည်။ )
The First Powerful Oral Drug For The Treatment of Covid-19
MOLNUPAC 400 mg contains Molnupiravir 400 mg and is indicated for the treatment of mild to moderate coronavirus disease 2019 (COVID-19) in adults with a positive SARS-COV-2 diagnostic test and who have at least one risk factor for developing severe illness.
Molnupiravir is a prodrug that is metabolised to the ribonucleoside analogue N-hydroxycytidine (NHC) which distributes into cells where it is phosphorylated to form the pharmacologically active ribonucleoside triphosphate (NHC-TP). NHC-TP acts by a mechanism known as viral error catastrophe. NHC-TP incorporation into viral RNA by the viral RNA polymerase, results in an accumulation of errors in the viral genome leading to inhibition of replication.
APPROVAL STATUS
Molnupiravir was approved by the UK's Medicines and Healthcare products Regulatory Agency as the world's first oral product to treat symptomatic Covid-19 1
Molnupiravir Receives U.S. FDA Emergency Use Authorization for the Treatment of High-Risk Adults With Mild to Moderate Covid-19 2
Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults 3
Indian drug regulator Central Drugs Standard Control Organization (CDSCO) has approved the anti-viral drug Molnupiravir for restricted emergency use against coronavirus 4
Indications
MOLNUPAC 200/400 is indicated for treatment of mild to moderate coronavirus disease 2019 (COVID-19) in adults with a positive SARS-COV-2 diagnostic test and who have at least one risk factor for developing severe illness.
Dosage and Administration
Adults (18 Years and older): The recommended dose of MOLNUPAC is 800 mg (two 400mg capsules) taken orally every 12 hours for 5 days. The safety and efficacy of molnupiravir when administered for periods longer than 5 days have not been established. MOLNUPAC should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Missed dose If the patient misses a dose of MOLNUPAC within 10 hours of the time it is usually taken, the patient should take as soon as possible and resume the normal dosing schedule. If a patient misses a dose by more than 10 hours, the patient should not take the missed dose and instead take the next dose at the regularly scheduled time. The patient should not double the dose to make up for a missed dose.
For oral use. MOLNUPAC capsules can be taken with or without food. The capsules should be swallowed whole with a sufficient amount of fluid (e.g., a glass of water). The capsules should not be opened, crushed or chewed.
Reference Links
1. https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra, https://www.tbsnews.net/bangladesh/health/eskayef-square-get-dgda-approvalproduce- covid-19-drug-molnupiravir-327271
2. https://www.merck.com/news/merck-and-ridgebacks-molnupiravir-receives-u-s-fda-emergency-use-authorization-for-the-treatment-of-high-risk-adults-with-mild-to-moderate-covid-19/
3. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain
4. https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/covid-19-corbevax-covovax-molnupiravir-approved-for-emergency-use/articleshow/88538268.cms,
| Molnupiravir | 400 mg |
CONTRAINDICATIONS
Hypersensitivity to the active substance or to any of the excipients. Interaction with other medicinal products and other forms of interaction No drug interactions have been identified based on the limited available data. No clinical interaction studies have been performed with molnupiravir.
FERTILITY, PREGNANCY AND LACTATION
Pregnancy: There are no data from the use of MOLNUPAC 200/400 in pregnant women. Studies in animals have shown reproductive toxicity. MOLNUPAC 200/400 is not recommended during pregnancy. Women of childbearing potential should use effective contraception for the duration of treatment and for 4 days after the last dose of MOLNUPAC 200/400 (molnupiravir).
Breast-feeding: It is unknown whether molnupiravir or any of the components of molnupiravir are present in human milk, affect human milk production, or have effect on the breastfed infant. Animal lactation studies with molnupiravir have not been conducted. Based on the potential for adverse reactions on the infant from MOLNUPAC 200/400, breast-feeding is not recommended during treatment and for 4 days after the last dose of MOLNUPAC 200/400.
Fertility: There were no effects on female or male fertility in rats at NHC exposures approximately 2 and 6 times respectively, the exposure in humans at the recommended human dose (RHD).
Undesirable effects
Summary of safety profile: In an interim analysis of a Phase 3 trial of subjects with mild to moderate COVID-19 treated with molnupiravir (n=386), the most common adverse reactions (≥1% of subjects) reported during treatment and during 14 days after the last dose were diarrhoea (3%), nausea (2%), dizziness (1%) and headache (1%) all of which were Grade 1 (mild) or Grade 2 (moderate).