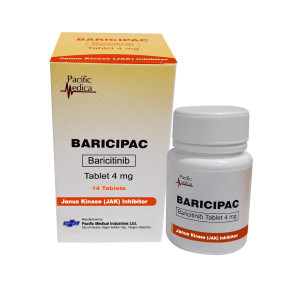
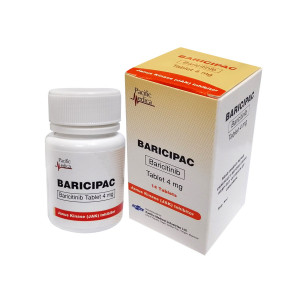
Baricipac
- Category: ဆေးရုံဆေးခန်းသုံးပစ္စည်းများ
- UOM: Bot of 14's Tablet
18,460 Kyats
BARICIPAC
This medicine is prescription only medicine (POM ) and required under medical supervision .
ဤဆေးသည် POM ဆေးအုပ်စုတွင်ပါဝင်သောကြောင့် ဆရာဝန်ညွှန်ကြားချက်အတိုင်းသာ သုံးစွဲသင့်ပါသည် ။
REMARK : Online order delivery may take from 3 to 5 days depend on area. For some areas in Yangon region such as North Dagon, South Dagon , Dagon Seikkan , Hlaing Thar Yar, Shwe Pyi Thar , Thanlyin , delivery will be around 1 week.
(မှတ်ချက် : အွန်လိုင်းမှတဆင့်မှာယူမှုများအတွက် အခမဲ့ပို့ဆောင်မှုမှာ ၃ ရက်မှ ၅ ရက်အတွင်းကြာမြင့်နိုင်ပါသည်။ အချို့ ဧရိယာများဖြစ်သည့် မြောက်ဒဂုံ ၊ တောင်ဒဂုံ ၊ ဒဂုံဆိပ်ကမ်း ၊ လှိုင်သာယာ ၊ ရွှေပြည်သာ ၊ သံလျှင် စသည့်မြို့များမှာမှာ တစ်ပတ်တရက်သာ ပို့ဆောင်သောကြောင့် ၁ ပတ်ခန့်ကြာမြင့်နိုင်ပါသည်။ )
Description
Baricitinib is a Janus kinase (JAK) inhibitoe indicated for the treatment of adult patients with moderately to severely active rheumaoid arthiritis who have had an inadquate response to one or more TNF antagonist therapies was intially approved y USFDA in 2018. On November 19, 2020, the US-FDA issued an Emergency Use Authorization (EUA) for emergency use of Baricitinib, in combination with remdisivir, for the treatment of suspected or laboratory confirmed coronavirus disease 2019 (COVID-19) in certain hospitalized patients requiring supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). US-FDA further reissued the Letter of Authorization on July 28, 2021, to no longer require that baricitinib be used in combination with remdesivir. Again on Decmber 20, 2021, US-FDA reissued the July 28, 2021 EUA of baricitinib for treatmment of COVID-19. Also, on 14 Jan 2022, WHO has strongly recommended the use of Baricitinib in patients with severe or critical COVID-19.
Action
Baricitinib is a reversible Janus kinase (JAK) inhibitor that is selective for JAK1 and JAK2, which are intracellular enzymes involved in the stimulation of haematopoiesis and immune cell function via a signalling pathway. The JAK/STAT pathway mediates the signaling of multiple cytokines and interruptionh this pathway is therefore a useful way to modulate the immunopathology seen with SARS-CoV-2 infection.
Indication
Treatment of COVID-19 in hospitalized adults and pediatric patients 2 years of age or older requiring supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO)
Moderate to Severe Rheumatoid Arthiritis
Severe Alopecia areata
Dosage and Administration
The recommended dosage for adults is 4 mg once daily orally for 14 days or until hospital discharge, whichever occurs first. Children above 9 years: 4 mg once daily orally for 14 days or until hospital discharge, whichever occurs first. Children 2-9 years old: 2 mg once daily orally, for 14 days or until hospital discharge, whichever occurs first. Can be taken with or without food.
Do not initiate treatment in patients with absolute lymphocyte count (ALC) <500 cells/mm3, absolute neutrophil count (ANC) <1,000 cells/mm3, or Hb level <8 g/dL. Dosing interruption or discontinuation may be required if serious infections or other serious adverse reactions (e.g. MI, DVT) occur, or according to the severity of the patient's laboratory abnormalities.
| Baricitinib | 4 mg |
Contraindications
Active, serious infections other than COVID-19, including localised infections and active TB. Pregnancy and lactation. Concomitant use with other biological DMARDs, strong immunosuppressants (e.g. azathioprine, ciclosporin, tacrolimus), and live vaccines.
Special Precautions
Patient with CV risk factors, known malignancy (apart from successfully treated non-melanoma skin cancer), risk factors for gastrointestinal perforation (e.g. history of diverticulitis); chronic or recurrent infection, underlying condition predisposing to infection; risk factors for DVT or pulmonary embolism (e.g. obesity, history of DVT or pulmonary embolism, undergoing major surgery, immobilisation). Current or past smokers. Patient who has been exposed to TB or who travelled or resided in areas where mycoses or TB are endemic. Patient taking potent organic anion transporter 3 (OAT3) inhibitors (e.g. probenecid). Moderate renal impairment. Children (when used for COVID-19) and elderly.
Adverse Reactions
Significant: Malignancies (e.g. lymphoma, non-melanoma skin cancer), reactivation of virus (e.g. herpes zoster, herpes simplex) or latent infections (e.g. TB), diverticulitis, gastrointestinal perforation, lymphocytopenia, anaemia, neutropenia, increased AST and ALT, lipid elevations (e.g. dose-related increases in total cholesterol, triglycerides, LDL and HDL cholesterol levels), hypersensitivity reactions (e.g. angioedema, urticaria, rash). Rarely, lymphoproliferative disorders.